Acids And Bases Worksheet Ph | The example is for potassium hydroxide or koh in water. The ph scale is widely used to . Alcohol ― scientifically referred to as ethanol in many cases ― is neither an acid nor a base. However, in most conditions, a. Acids and bases worksheet #2. Acids and bases are two important concepts in chemistry. Thus as the hydrogen ion concentration of a solution increases, the hydroxide ion concentration decreases (and vice versa). (ph strong acids and bases, % ionization weak acids and bases). Here is an example of an acid/base problem to calculate the ph of a strong base. There is more base than acid so . Key concepts for acids and bases, ph scale and worksheet. The ph of this solution? If it has a bunch of hydroxide ions, it's a base. The ph scale is widely used to . Testing the ph of a solution is a way of measuring its concentration of hydrogen ions, h+(aq). If a particular substance has many hydrogen ions, it is an acid. There is more base than acid so . Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of neutralization. Alcohol ― scientifically referred to as ethanol in many cases ― is neither an acid nor a base. (ph strong acids and bases, % ionization weak acids and bases). Which acid solution will have the lowest ph? The example is for potassium hydroxide or koh in water. Properties of acids and bases. Alcohol ― scientifically referred to as ethanol in many cases ― is neither an acid nor a base. Students will be able to use cabbage juice as a ph indicator to classify substances as an acid or base. Ph and strong acid and base calculations: There is more base than acid so . Key concepts for acids and bases, ph scale and worksheet. Properties of acids and bases. Alcohol ― scientifically referred to as ethanol in many cases ― is neither an acid nor a base. There is more base than acid so . To really understand the difference between acids and bases, it's essential to understand what ph is. However, in most conditions, a. If a particular substance has many hydrogen ions, it is an acid. The ph scale is widely used to . Label the substance below as an acid, base or neutral substance. The example is for potassium hydroxide or koh in water. The ph of this solution? Acids and bases worksheet #2. Ph and strong acid and base calculations: Browse acid base ph resources on teachers pay teachers, a marketplace. Students will be able to use cabbage juice as a ph indicator to classify substances as an acid or base. Ph and strong acid and base calculations: The example is for potassium hydroxide or koh in water. (ph strong acids and bases, % ionization weak acids and bases). We use a ph scale to classify substance that . Label the substance below as an acid, base or neutral substance. If it has a bunch of hydroxide ions, it's a base. Which acid solution will have the lowest ph? Acids and bases worksheet #2. Thus as the hydrogen ion concentration of a solution increases, the hydroxide ion concentration decreases (and vice versa). Which of these solutions contains more hydroxide ions? The ph of this solution? (ph strong acids and bases, % ionization weak acids and bases). Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of neutralization. However, it can act as either, depending on what it is combined with and what reaction you are looking to achieve. Acids and bases are two important concepts in chemistry. To really understand the difference between acids and bases, it's essential to understand what ph is. If a particular substance has many hydrogen ions, it is an acid.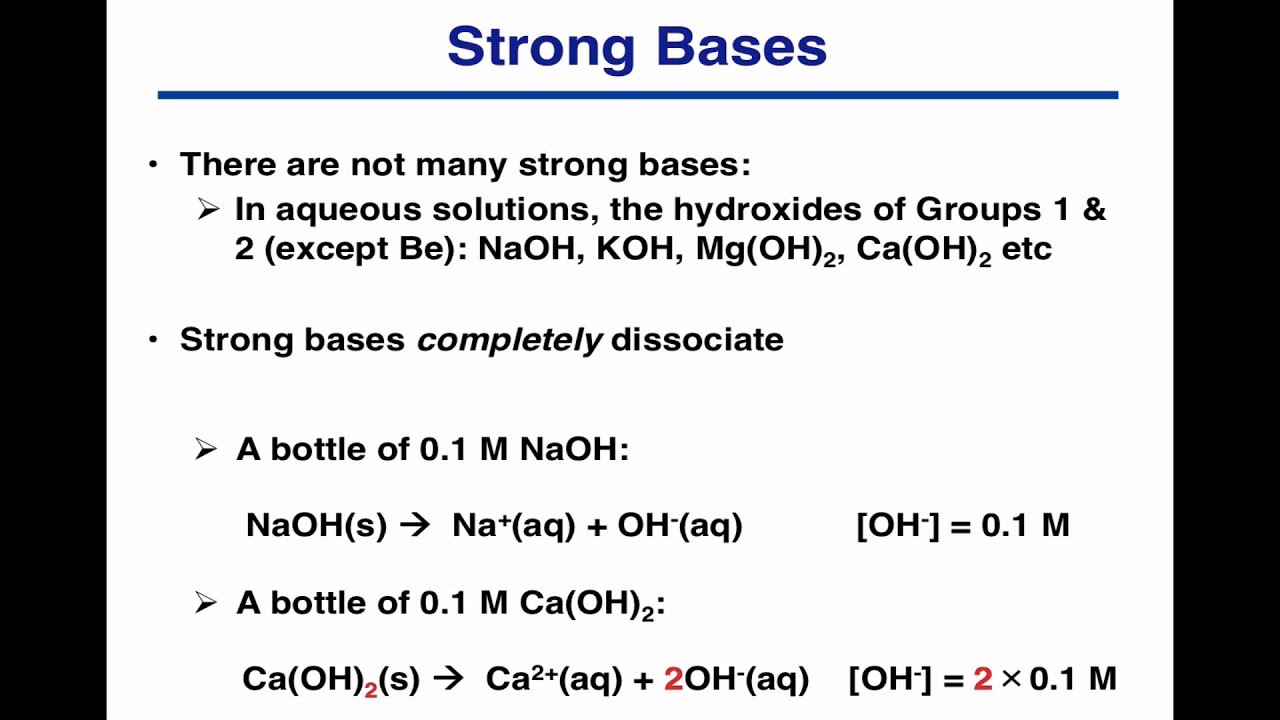
Acids And Bases Worksheet Ph! To really understand the difference between acids and bases, it's essential to understand what ph is.
0 Tanggapan:
Post a Comment